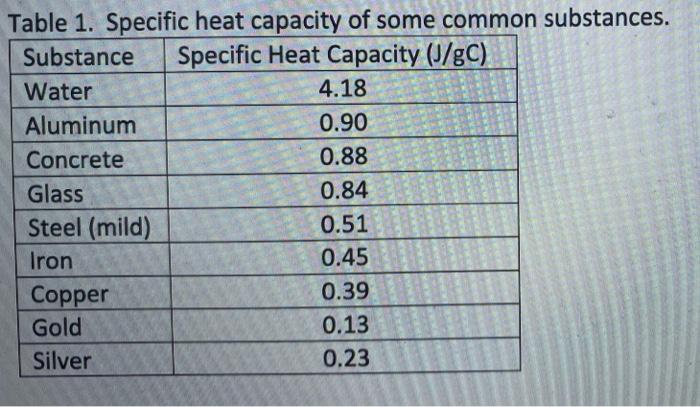
Calculate the energy required to heat 790.0g of iron from −2.6°C to 14.9°C. Assume the specific heat - brainly.com

Comparison between the calculated and experimental heat capacity of... | Download Scientific Diagram

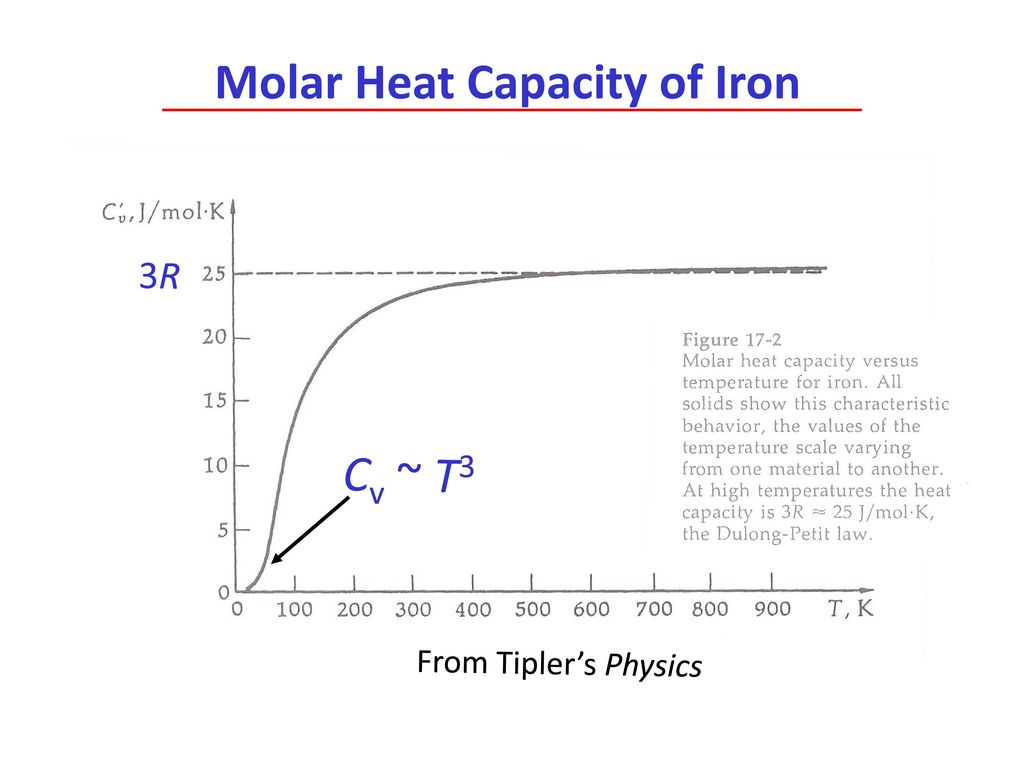
Molar heat capacity of iron (at mass 56) will be ........... Times its specific heat. | CLASS 11... - YouTube
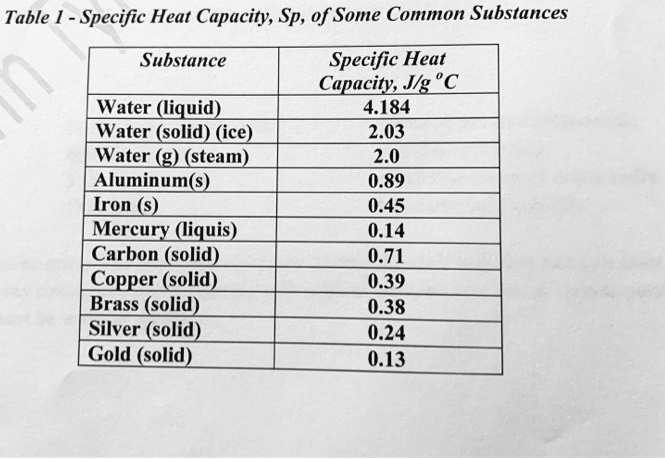
SOLVED: Table 1: Specific Heat Capacity, Sp, of Some Common Substances Substance Specific Heat Capacity, J/g 4.184 2.03 2.0 0.89 0.45 0.14 0.71 0.39 0.38 0.24 0.13 Water (liquid) Water (solid) (ice)

Derived mean values of the specific heat of pure iron in comparison... | Download Scientific Diagram

Calculate the amount of heat required to raise the temperature of 5 g of iron from `25^(@)C \"to\" - YouTube
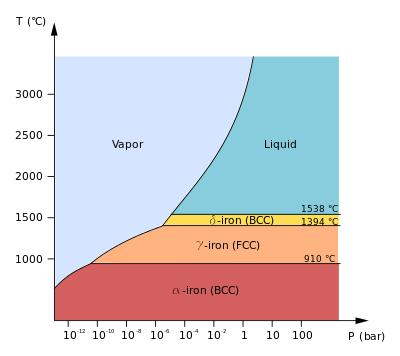
Molten iron is extremely hot, averaging about 1,500 C. The specific heat of iron is 0.46 J/gC. How much heat is released to the atmosphere when 1 kg molten iron cools to